Contact person: Dimitrios Avgoulas (dimiavgou@gmail.com)
Biofilms inside water systems are heterogenous structures, where water pores and channels surrounding cell clusters are crucial features that govern cells viability, and biofilm morphology and resilience, as well. The scope of this project is to tailor biofilm structures based on their response to bulk and boundary hydrodynamic conditions in water systems, i.e., flow profiles, wall shearing action. This may lead on one hand to less adherent/coherent, and so less resilient, biofilms in water systems, e.g. in potable water pipes, condensation units etc, and on the other hand to more functional biofilms for nutrients production and environmental remediation, especially during future long term missions [1].
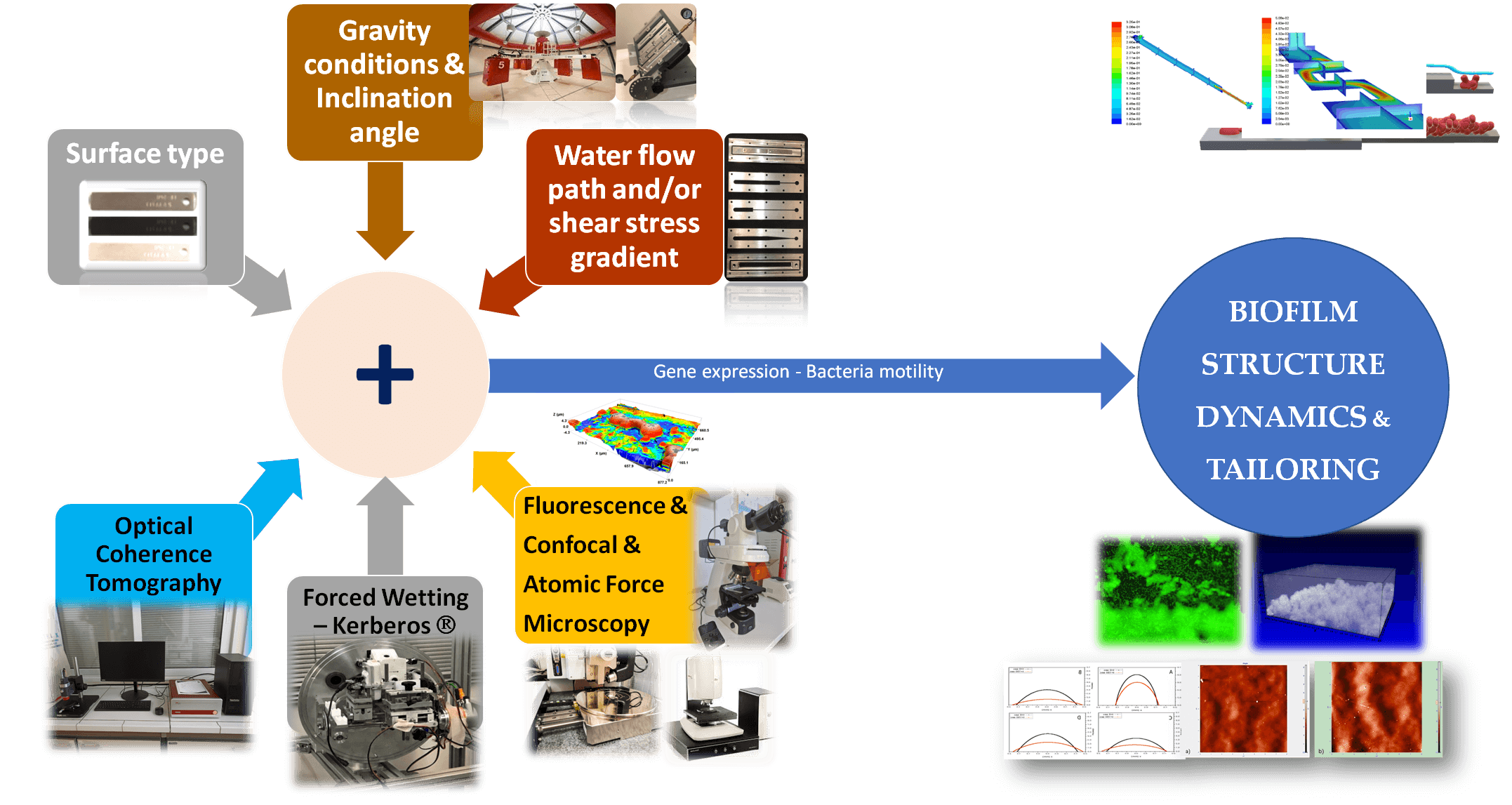
To this aim, development of biofilm morphology inside water systems, in particular the mechanisms for the formation of water channels and pores, along with their temporal and spatial distribution, should be elucidated. Channels and pores are the main pathway both for the convective nutrients transport meant to augment biofilms and for the permeation of antimicrobial solutions meant to destroy biofilms [2]. To this end, the project aims to examine the evolution of the 3D structure transformations of biofilms in continuous water flow systems under real world conditions, e.g., using flow profiles and boundary walls employed in industry and in ISS.
The primary tool to register in real-time such 3D structure dynamics will be Optical Coherence Tomography (OCT), a technique that allows accurate on-line monitoring of biofilms growth [3]. Biofilm samples at different phases of transformation will be also examined by confocal, fluorescence and/or atomic force microscopy for cross-inspection with OCT results. The external morphology of biofilms will be also assessed by Kerberos®, a device facilitating forced wetting experiments [4-6]. The obtained data will be utilized to confirm and/or expand established theoretical approaches regarding biofilm development. Custom-made experimental flow cells will be used to investigate the effect of different: a) flow path geometry (velocity and shear stress gradients), b) type of metallic and polymeric wetted surfaces used in industry and in ISS water systems, c) inclination of wetted surfaces (varying gravity component). The experimental set-up will be based on detailed 3D CFD simulations.
Research questions:
What are the 3D structure transformations during the evolution of biofilms at solid-liquid interfaces and how they are affected by the flow geometry, the wetted surface type, and the inclination?
Is it possible to guide/control/patronize/manipulate biofilm structures using mechanical means?
References
[1] Landry K.S., Morey J.M., Bharat B., Haney N.M., Panesar S.S. Biofilms-Impacts on Human Health and Its Relevance to Space Travel. Microorganisms 8(7) (2020):998.
[2] Quan K., Hou J., Zhang Z., Ren Y., Peterson B.W., Flemming H.-C., Mayer C., Busscher H.J., van der Mei H.C. Water in bacterial biofilms: pores and channels, storage and transport functions, Critical Reviews in Microbiology 48:3 (2022), 283-302.
[3] Li M., Matouš K., Nerenberg R. Data-driven modeling of heterogeneous viscoelastic biofilms. Biotechnology and Bioengineering 119, (2022) 1301– 1313.
[4] Rios-Lopez I., Evgenidis S., Kostoglou M., Zabulis X., Karapantsios T. Effect of initial droplet shape on the tangential force required for spreading and sliding along a solid surface. Colloids and Surfaces A: Physicochemical and Engineering special issue. 549 (2018) 164-173.