Contant Persons: C. Koukiotis [koukiotis@gmail.com]
Emulsions are complex soft-materials, where fluid is dispersed into a liquid matrix, with interesting industrial applications in a variety of products, such as foods and cosmetics. Emulsions are multi-component dispersed systems consisting of two immiscible phases, a non-polar and a polar one, containing soluble or dispersed macromolecules, such as different kinds of emulsifiers. These systems tend to separate into distinct phases, especially in liquid-type products, which is why emulsions’ stabilization and destabilization concern modern technology. Emulsions’ stability against phase separation during storage is critically dependent on the type of the emulsifying and stabilizing agents present at the interfaces and the distribution of the dispersed phases.
Emulsions’ stability can be investigated by several methods:
- intrusive:
- techniques performing measurements on withdrawn samples, etc.
- non-intrusive:
- optical observation determining droplets’ size distribution (e.g. cameras)
- emulsions’ electrical conductance (IVED) or conductivity (tomography) measurements in order to determine the spatial fraction and distribution of the two phases over a large area inside an opaque dispersion. During emulsification, the initial large oil droplets disintegrate into smaller ones due to shearing activity of mixing, water continuously disperses in the intra-droplet spaces and the electrically accessible water becomes progressively less, reflecting the structural changes during emulsification. The inverse process takes place when the mixing stops, the drops progressively gather in agglomerates and the emulsion starts to destabilize.
In order to comprehend the correlation between emulsions’ stability and the system’s interfacial properties, this project examines:
- the stability of emulsions using a novel electrical conductance tomography technique which allows to identify the longitudinal volume fraction and phase distribution along a test vessel (Fig. 1, Fig. 2)
- the interfacial properties of emulsions (static and dynamic interfacial tension, interfacial rheology, dilatational viscosity and elasticity) with in-house and commercial equipment such as PAT- 1S (Sinterface, Fig. 3), ODBA-1 (Sinterface, Fig. 4), BPA-1S (Sinterface, Fig. 5), TE2 (Lauda, Fig. 6), TVT 2 (Lauda, Fig. 7), etc.
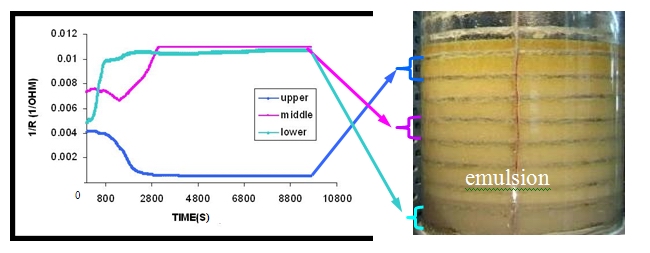
Figure 1: Time stability of sunflower oil/water (50/50) and 3% Soybean Protein Isolate emulsion at different positions inside the test vessel as measured by the custom made electrical technique.
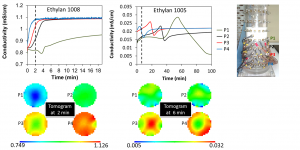
Figure 2: Difference in phase separation of oil-to-water emulsions with surfactant ethylan 1008 and ethylan 1005.

Figure 3: Profile analysis tensiometer (PAT-1, Sinterface)

Figure 4: Oscillating Drop and Bubble Analyser (ODBA-1, Sinterface)

Figure 5: Maximum bubble pressure tensiometer (BPA-1S, Sinterface)

Figure 6: Ring and plate tensiometer (TE 2, LAUDA)

Figure 7: Drop volume tensiometer (TVT 2, LAUDA)
This project also studies emulsions’ phase inversion as low-energy emulsification method which produces stable emulsions. Phase inversion can be obtained by changing the water volume fraction (emulsion inversion point method) by successively adding water into the mixture of oil and surfactant (Fig. 8).
![Figure 8: Representation of emulsion phase inversion method [Image taken from McClements DJ. Soft Matter 2011;7:2297].](http://karapant.webpages.auth.gr/wp-content/uploads/2015/01/Figure-8-300x232.png)
Figure 8: Representation of emulsion phase inversion method [Image taken from McClements DJ. Soft Matter 2011;7:2297].
This technique has successfully been applied in studies to produce:
- stable nanoemulsions of an aminopolysiloxane (Fig. 9). Particle size distributions are measured by DLS (Fig. 10).
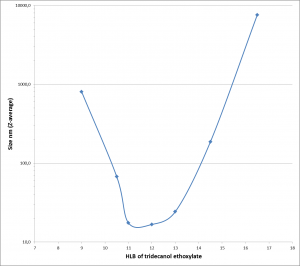
Figure 9: Z-average particle size of polysiloxane emulsion by DLS vs the HLB of the used C13 ethoxylated surfactan
- paraffin oil emulsions with two non-ionic surfactants, Ethylan 1005 and Ethylan 1008, which have different chemical structure. Emulsification takes place using 20% of paraffin oil, 75% of Millipore water and 5% of pure surfactant or mixture of the two surfactants in different proportions. Destabilization of the emulsions over time is observed with non-intrusive methods (e.g. cameras) and with the use of in-house Axiostar Plus (Carl Zeiss Inc., Fig. 11) microscope, the correlation between emulsion stability and size of droplets is under research. Special attention is given to the emulsions prepared with pure surfactants, as data analysis has shown that the emulsion with Ethylan 1005 consists of small sized droplets (1-50μm) but shows low stability, while the emulsion with Ethylan 1008 consists of larger droplets (1-300μm) but shows better stability (Fig. 12, Fig. 13, Fig. 14). In order to understand this phenomenon, interfacial properties were tested with in-house equipment such as PAT-1S and TE2 and a research on steric phenomena and theory is in progress.
Figure 11: Microscope equipped with a camera and image analysis software (Axiostar Plus, Carl Zeiss Inc.)
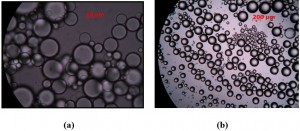
Figure 13: Photos under microscope of paraffin oil emulsions in water with surfactants (a) Ethylan 1005, (b) Ethylan 1008.
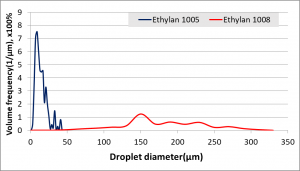
Figure 14: Comparison of Droplet Volume Distribution for parafin oil emulsion in water with Ethylan 1005 & Ethylan 1008 surfactants.

