Contact Person: Yiota Xanthopoulou [giotaxanthopoulou95@gmail.com]
Among the different nucleate boiling heat transfer enhancement techniques, the use of additives such as surfactants for liquids appears to be quite viable as a boiling heat transfer enhancement technique and has attracted a lot of research over the past decades. Of the available studies, aqueous surfactant solutions are quite common because the surface tension of water is greater than that of many liquids with amphiphilic structures. Addition of surfactants to water reduces the surface tension of the aqueous surfactant solutions considerably. The reduction in the surface tension is dependent upon several factors such as surfactant bulk concentration, surfactant type and molecular weight, solution temperature, interfacial conditions and so on. When the surfactant concentration reaches a critical value, the surface tension tend to a constant value which corresponds to the critical micelle concentration (cmc) for each surfactant. For the boiling phenomena of aqueous surfactant solutions, knowledge of the dynamic surface tension and the corresponding adsorption behavior at boiling temperature is very crucial to understanding the boiling phenomena (Cheng et al. 2007)
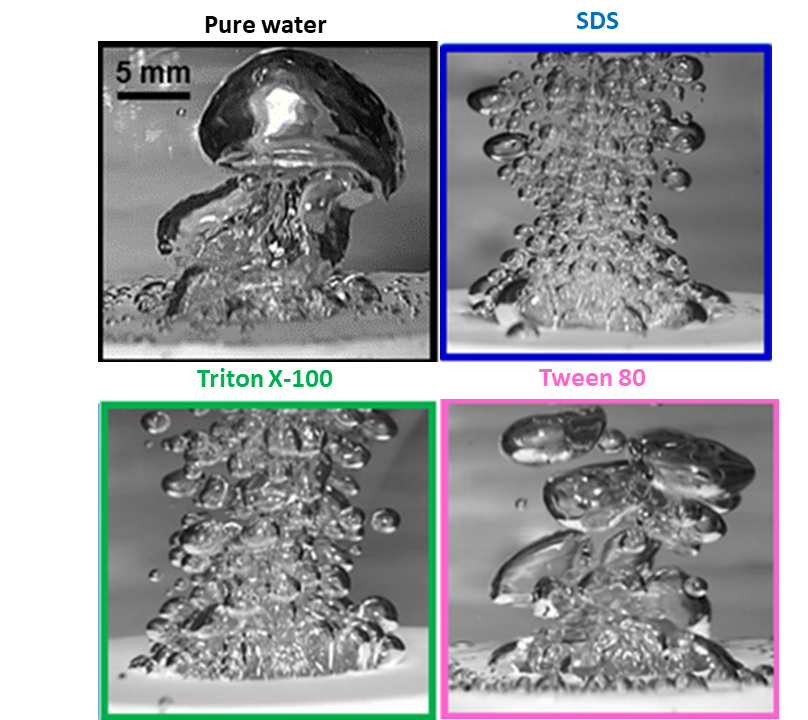
Addition of a small amount of surfactant in pure water results in modification of the bubbles’ size, number and behavior, decreasing the bubble size and increasing their number (Fig.1, Fig.2).
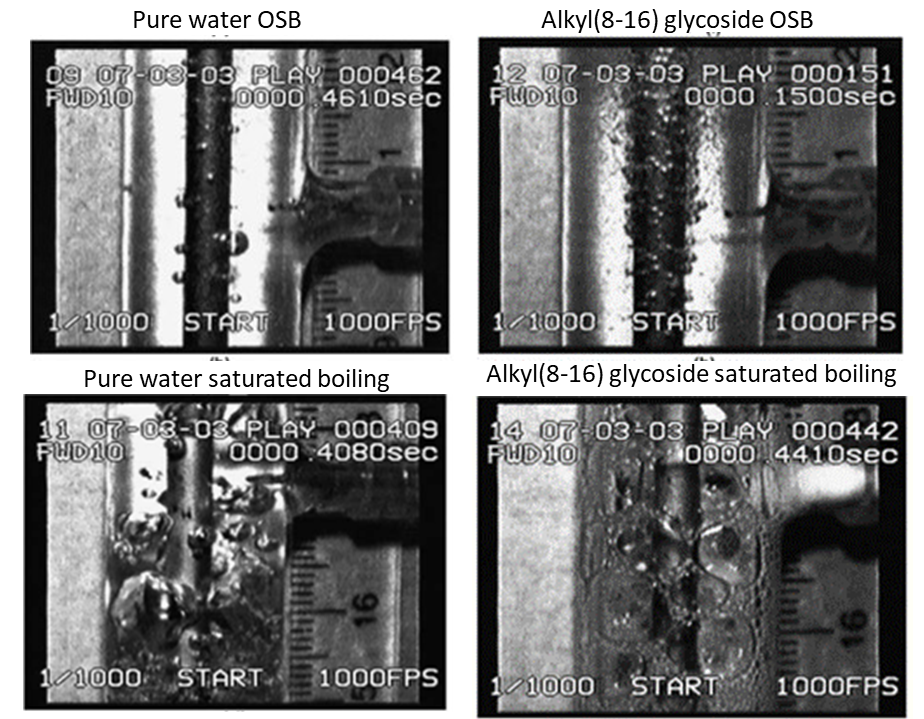
Fig. 1. Flow boiling of Alkyl (8–16) Glycoside (300 ppm) in comparison with flow boiling of pure water (Hestroni et al. 2004)
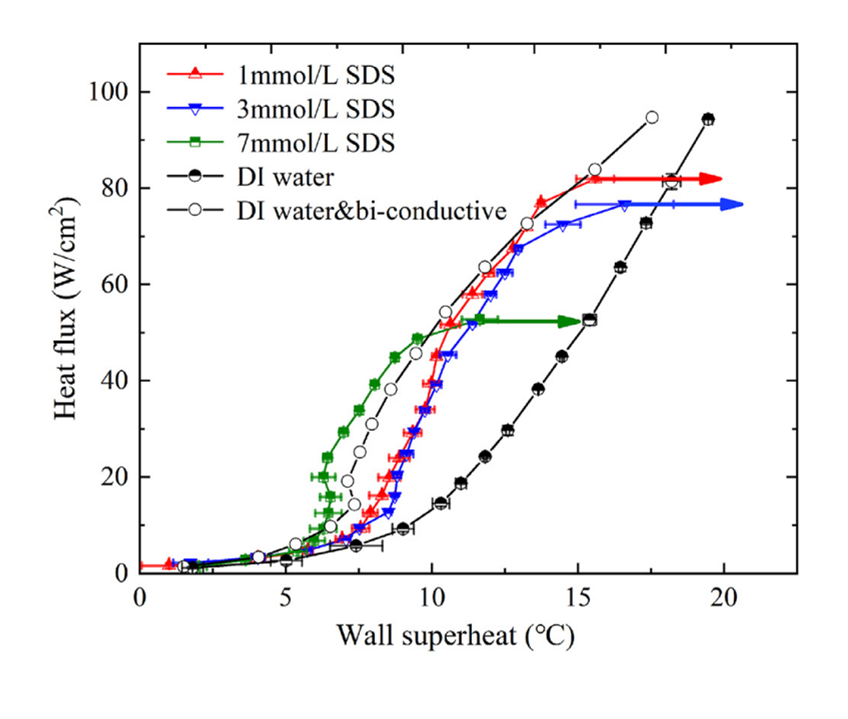
Vapor bubbles appear to be smaller in size and steadier with less active interactions i.e. decreased coalescence tendency. The rate of nucleus formation is proportional to e^(-σ^3 ). Thus, small decreases in σ should cause large increases in the number of nuclei. (Wu et al. 1995) Under these conditions the heat transfer coefficient increases in saturated boiling of surfactants compared to that in pure water.(Fig.3, Fig.4) Because of their low concentration, the presence of surfactants in water causes no significant change in the solvent physical properties except for surface tension, whereas the presence of surfactants at higher concentrations in water may causes big change of the viscosity in the solvent (non-Newtonian fluidic behavior).
Heat exchangers with small channels are prevalent in industries such as electronic, transport, and chemical processing. The surface tension force is important and might affect flow patterns and heat transfer in such channels. (Hestroni et al. 2004). Studies have focused particularly on nucleate pool boiling of various aqueous additive solutions. (Cheng et al. 2007) There are only a few studies on flow boiling with surfactant additives.
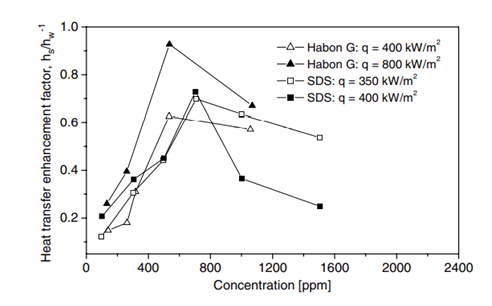
Fig. 3. Heat transfer enhancement versus concentration for Habon G solution (Hestroni et al. 2001 ) and for SDS (Tzan and Yang 1990)
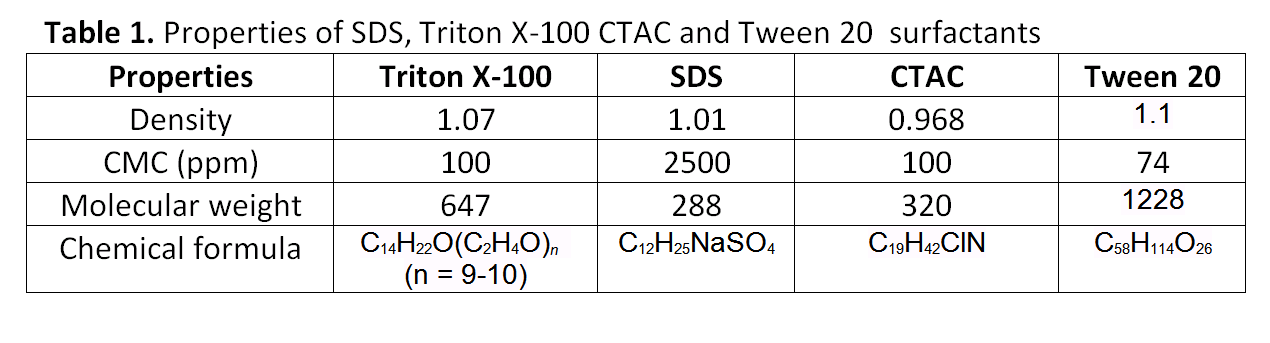
This project aims to investigate the behavior of aqueous SDS, Triton X-100, Tween 20 and CTAC solutions during experiments of flow boiling in dieferent concentrations, i.e. dieferent surface tension conditions and the influence of these surfactant additives in boiling heat transfer. Properties of these surfactants are presented in table 1. The effect of temperature in surface tension will be also studied. To investigate bubble behavior during these experiments, videos and pictures will be recorded using a high speed camera (Photron Multi FASTCAM) (Fig.5). Figure 6 shows the experimental apparatus.

Fig.5 Video: flow boiling of pure water. Mass flux: 300 kg/m2s, Heat flux: 750 kW/m2. Lens: Nikon micro nikkor 60mm
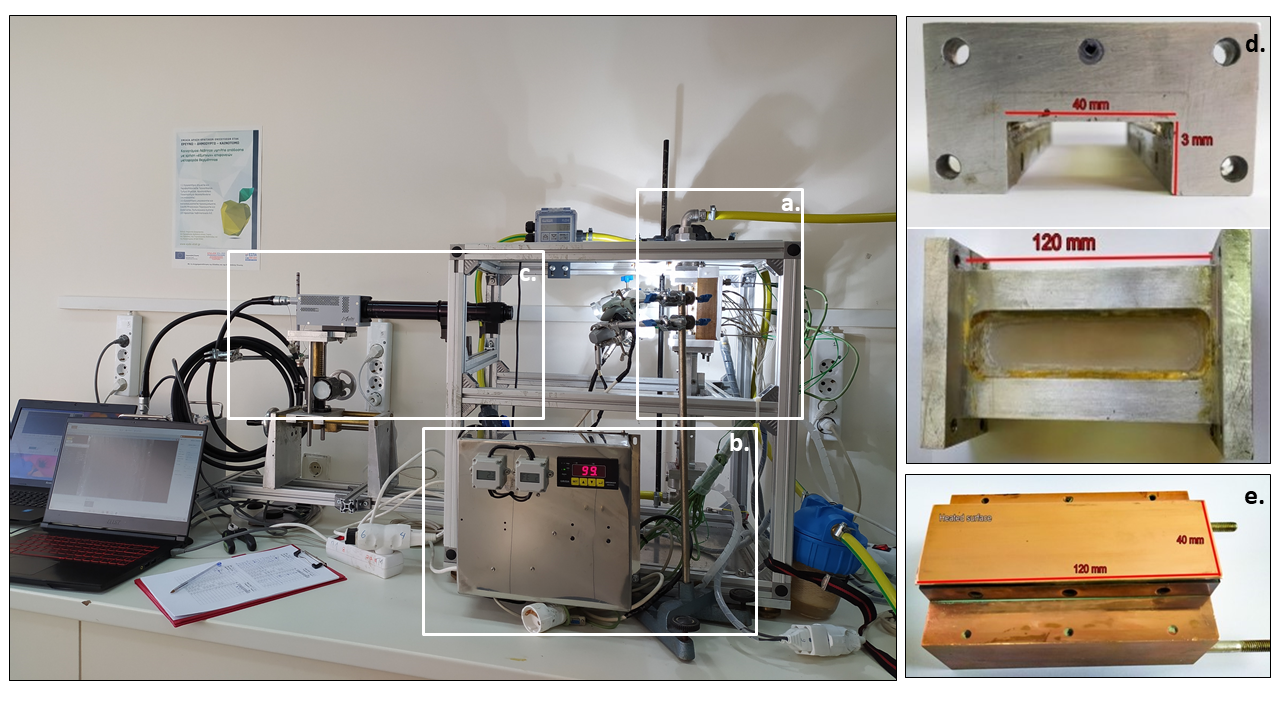
Fig. 6. Experimental apparatus used in the project. a. Test section, b. Temperature control panel, c. High-speed camera, d. Test section-channel, e. Test section-heating surface
References
- Cheng, D. Mewes and A. Luke (2007), Boiling phenomena with surfactants and polymeric additives: A state-of-the-art review, International Journal of Heat and Mass Transfer 50, 2744–2771
- Hetsroni, M. Gurevich, A. Mosyak, R. Rozenblit, L.P.Yarin, (2002a). Subcooled boiling of surfactant solutions. Int. J. Multiphase Flow 28, 347–361.
- G.Hetsroni, J.L. Zakin, Z. Lin, A. Mosyak, E.A. Pancallo, R.Rozenblit (2001b) The effect of surfactants on bubble growth, wall thermal patterns and heat transfer in pool boiling. International Journal of Heat and Mass Transfer 44, 485–497.
- Hetsroni, J.L. Zakin b, M. Gurevich, A. Mosyak, E. Pogrebnyak and R. Rozenblit (2004), Saturated flow boiling heat transfer of environmentally acceptable surfactants, International Journal of Multiphase Flow 30, 717–734
- M.Q. Raza et al. (2018), Wettability-independent critical heat flux during boiling crisis in foaming solutions, International Journal of Heat and Mass Transfer 126, 567–579
- W.T. Wu, Y.M. Yang, J.R. Maa, (1995), Enhancement of Nucleate Boiling Heat Transfer and Depression of Surface Tension by Surfactant Additives , J. Heat Transfer, 117, 526-529
- Y.L. Tzan, Y.M. Yang (1990), Experimental study of surfactant effects on pool boiling heat transfer, J. Heat Transfer 112 207–212
- J.Yin, X. Xiao, L.Feng, K. Zhong , H.Jia (2020), Experimental investigation of pool boiling characteristics of surfactant solutions on bi-conductive surfaces, nternational Journal of Heat and Mass Transfer 157, 119914