Contact person: Petala M. [petala@civil.auth.gr]
The aim of this work is to determine the most important components of biofilm matrix. Biofilms are matrix-enclosed cells of microorganisms (e.g. bacteria) that can colonize in a wide range of surfaces (abiotic and biotic). The matrix is composed by bacteria themselves and is called EPS (extracellular polymeric substances). The major components are water, exopolysaccharides and other excreted cellular products, such as proteins, nucleic acids, humic substances and molecules that are responsible for the communication between bacterial cells. EPS is responsible for the three-dimensional architecture of the biofilm and also for adhesion to surfaces and for cohesion. Its form can vary and depends on the kind of microorganisms, the temperature, the availability of nutrients and the shear stress. In most cases, the composition of EPS affects considerably the mechanical properties of the biofilm and therefore influences the biofilm stability.
In this study, the impact of shear stress on the composition of biofilm matrix is investigated on two different materials: glass and low carbon stainless steel (SS 316L). Single species biofilm of Pseudomonas fluorescence is developed under well-defined shear stress conditions that are created by applying rotation in a jacketed rotating biofilm reactor (Figure 1). In Figure 2, the photos depict different morphologies of biofilms on glass coupons, with respect to rotation direction.
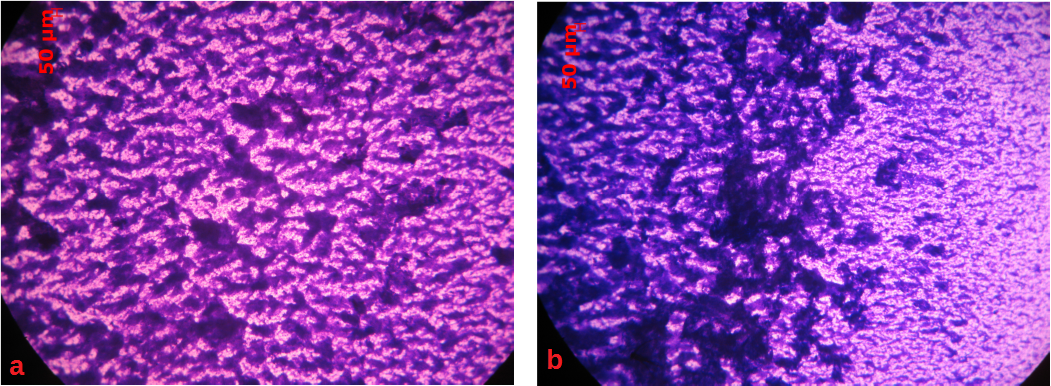
Figure 2: Optical microscope photos from a glass coupon, a: left side of the coupon (the downstream one), b: right side of the coupon (the upstream one). Scale bar: 50μm.
Parameters evaluated:
- Biofilm growth evaluation
- Biofilm morphology
- EPS: polysaccharides and proteins
Methods:
- Chemical analyses
- Optical Microscopy
References:
- Manuel Simões, Maria O. Pereira et al (2007), The effect of hydrodynamic conditions on the phenotype of Pseudomonas fluorescens biofilms, Biofouling: The Journal of Bioadhesion and Biofilm Research, 23:4, 249-258
- Dubois M, Gilles KA et al (1956). Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
- Lowry, O.H., Rosebrough, N.J., et al (1951), Protein measurement with the Folin Phenol Reagent, Biol.Chem 193: 265
- B. Fr¢lund , T. Griebe, P. H. Nielsen (1995), Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 43:755-761
